2021年上半年盘点:FDA批准52款新药,6款生物制品
2021-07-04 药智网 药智网
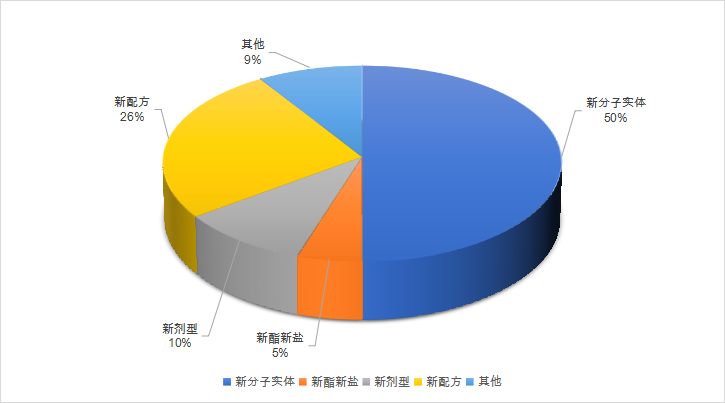
2021年1月至6月,美国FDA共计批准了52款新药,6款生物制品(CDER批准)。按照企业提交的申请分类包括新分子实体类占比50%,新酯新盐类或其他非共价键的衍生物类5%,新剂型10%,新配方26%
2021年1月至6月,美国FDA共计批准了52款新药,6款生物制品(CDER批准)。按照企业提交的申请分类包括新分子实体类占比50%,新酯新盐类或其他非共价键的衍生物类5%,新剂型10%,新配方26%,其他类(新药物组合等)9%。近半年来,其批准的新药药理主要分布在系统用抗感染药类、神经系统类、心血管系统类以及消化道代谢和抗肿瘤药等领域。
其中,FDA批准了共22款抗癌新疗法,包括靶向,免疫检查点抑制剂,过继性细胞免疫疗法等等,覆盖了几乎全部的癌症种类,值得一提的是KRAS和EGFR20ins两大难治性靶点打破了治疗僵局,迎来了首款靶向药物,又有很多幸运的病友们等来了新的希望和治疗选择。详细见:2021年上半年盘点:FDA批准抗肿瘤药物汇总
其中,商品名称MAVYRET和EPCLUSA这两款新药为美国FDA首次批准,都为新剂型类药物,并已获得了FDA的孤儿药认定。MAVYRET是格卡瑞韦和哌仑他韦的固定剂量组合药物,由艾伯维制药持有并于2021年6月获得FDA的上市批准,主要治疗慢性丙型肝炎病毒。EPCLUSA是一种核苷酸类似物聚合酶抑制剂和泛基因型NS5A抑制剂的固定剂量组合药物(索磷布韦和维帕他韦),由吉利德科学公司持有并于2021年6月获得FDA的上市批准,同样也用于慢性丙型肝炎病毒的治疗。
中国药企在2021年上半年中,有超过20家企业拿到了FDA授予的上市批准,有30个品种(多以注射剂和片剂为主)通过了FDA仿制药上市批准,同比下降16%。复星医药执掌牛耳,共计有9款仿制药在美国获批上市。
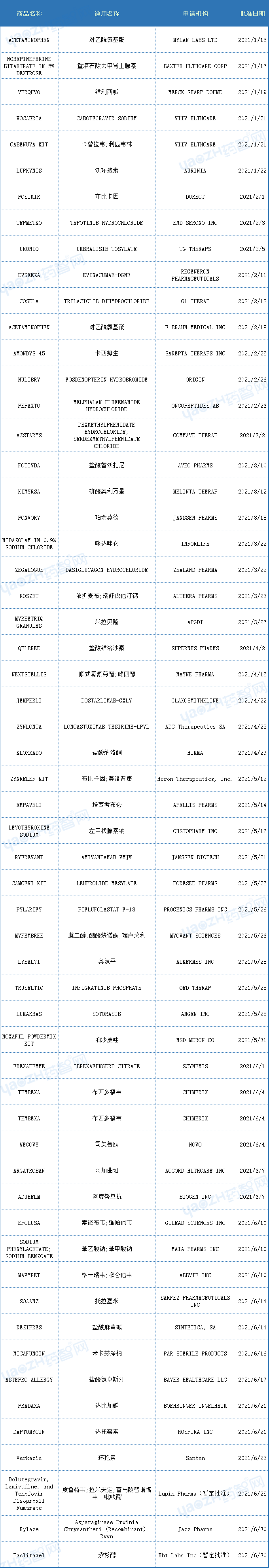
以下为2021年上半年FDA批准新药和部分生物制品的数据(参考)。
本网站所有内容来源注明为“williamhill asia 医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于williamhill asia 医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“williamhill asia 医学”。其它来源的文章系转载文章,或“williamhill asia 号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与williamhill asia 联系,williamhill asia 将立即进行删除处理。
在此留言












#生物制品#
81
👏👏👏
92
#FDA批准#
54
#FDA#批准#创新药#记录
0
学习了
96