Natl Sci Rev:蒲慕明院士撰文解读大型脑科学计划往何处去
2014-06-08 MedSci MedSci原创
关于大脑活动背后的神经回路,以及神经信息是怎样处理、储存和检索的,人类现有的知识还十分有限。用超级计算机模拟人脑需要大量的猜测,这可能使得模拟出的结果具有误导性,或者偏离实际。弄清每个神经元的活动以及所有神经元之间的连结性,具有十分重要的意义,但是对于充分了解大脑来说还只是其中的一小步。 美国总统和欧盟委员会于2013年分别公布了大型脑科学计划(BRAIN Initiative和Human
关于大脑活动背后的神经回路,以及神经信息是怎样处理、储存和检索的,人类现有的知识还十分有限。用超级计算机模拟人脑需要大量的猜测,这可能使得模拟出的结果具有误导性,或者偏离实际。弄清每个神经元的活动以及所有神经元之间的连结性,具有十分重要的意义,但是对于充分了解大脑来说还只是其中的一小步。
美国总统和欧盟委员会于2013年分别公布了大型脑科学计划(BRAIN Initiative和Human Brain Project),提出发展创新性的神经科学技术的新举措。大型脑科学计划的启动,说明很多政府已将人脑研究提高到国家议程的层面。全球共同推进对人类大脑结构和功能的理解,加深对感知、行为以及意识的研究,其成果有望帮助了解阿尔茨海默病和帕金森氏症等疾病,并为各种神经和精神疾病的治疗提供新的途径。
大型脑科学计划必然会促进有关测量神经元活动和神经连结性新技术的发展,以提高测量的时空分辨率和效率,通过更有效的人机界面探索脑活动的动力学,控制大脑回路的输出。中国科学院神经科学研究所蒲慕明院士特撰写观点文章“大型脑科学计划往何处去?”对此进行评介和展望,该文已在《国家科学评论》创刊号发表。
原始出处:
Poo mm. Whereto the mega brain projects? Natl Sci Rev (March 2014) 1 (1): 12-14.
英文原文:
Whereto the mega brain projects?
The mega brain projects announced by the US President and European Commission in 2013 offer new initiatives for developing innovative neurotechnology, but the prospect of effective therapies for brain disease remains uncertain.
In his 2013 State of the Union address President Obama of the United States shook the global neuroscience community by announcing a 10-year, 3 billion dollar project, the ‘Brain Activity Map’, that aims to ‘image every spike from every neuron’. This was followed on 2 April 2013 by an announcement from the White House of the BRAIN (Brain Research through Advancing Innovative Neurotechnologies) Initiative that promises multi-agency support for the development of new technologies for studying the brain [1]. On the other side of the Atlantic, the European Commission announced on 28 January 2013, that the ‘Human Brain Project’ (HBP) had been chosen as one of two ‘FET Flagship’ projects with 10-year funding of 1 billion Euros and the aim of simulating the human brain with supercomputers to better understand how the brain works and to diagnose brain disorders [2].
In comparison with the total government and private investment in brain research around the globe, the new funding proposed for these mega projects is relatively small. At the US National Institutes of Health alone, the budget for neuroscience-related intra- and extramural research amounts to 5 billion in the current year, 50 times larger than the first-year budget of 100 million promised for the BRAIN Initiative, although an increasing budget in subsequent years is expected. The private foundation support for neuroscience research has also been substantial. Nevertheless, what excites the global neuroscience community is not the amount of new funding available, but the fact that many governments are putting brain research high on their national agenda.
The recent surge in multi-governmental interest in brain research is by no means a coincidence. The societal burden of brain disorders, including neurological and psychiatric diseases, and substance abuse, now exceeds that of cardiovascular disease and cancer in both advanced and developing countries [3]. The statistics on brain disorders are alarming. Depression affects up to 10% of the population in many societies, and with increasing longevity, Alzheimer's disease affects 13% of the population at age 65, and nearly half of people above age 85. However, this crisis in medical care for brain disorders has not been met by advances in effective treatments, particularly for mental disorders and neurodegenerative diseases. Therefore, have the mega brain projects arrived in time to rescue us from this crisis?
FROM MAPPING TO UNDERSTANDING
Since the celebrated ‘Decade of the Brain’ in the 1990s, our understanding of the brain has progressed with unprecedented pace, from the genetic and molecular basis of neural development, to the synaptic and neural-circuit mechanisms for sensory and motor functions, and even higher cognitive functions such as learning and memory. The cause and genetic susceptibility of many neurological and neuropsychiatric diseases are increasingly clarified. These advances were made possible by new molecular tools derived from molecular and genomic biology, and the advent of brain imaging technologies. Magnetic resonance imaging provides a wide variety of non-invasive approaches for probing the structure and function of the human brain at the macroscopic level, from the connectivity of nerve tracts, to the activities of various brain regions during cognitive tasks, and the regional concentrations of various metabolites, transmitters and their receptors in the living brain. At the microscopic level of neurons and neural circuits, new developments in multi-photon microscopy and optogenetic approaches allow researchers to monitor and manipulate neuronal activity in animal model systems with cell-type specificity. In principle, it is now possible to know not only whether a specific group of neurons is active but also if they play a causal role in a particular brain function.
The structure complexity of the brain imposes a formidable task for deciphering the neuronal basis of brain functions at the microscopic level. The human brain consists of 100 billion neurons of hundreds of different types, with 1015 specific connections among them that form neural networks underlying diverse brain functions. The best current technology allows simultaneous monitoring of activities from about a 1000 neurons. Scaling up the number of neurons by a few orders of magnitude, while still maintaining single-cell resolution, requires the development of new sensors for neuronal activity as well as new in vivo imaging and analysis methods. The roadmap set by the original proponents of the BRAIN Initiative was realistic [4], stating that a complete activity map could be achieved for the nematode worm (containing 302 neurons), and a part of the fruit fly's brain (∼15 000 neurons) in five years. The entire fly's brain (∼135 000 neurons) could be mapped in 10 years, and perhaps the entire mouse neocortex (tens of millions of neurons) in 15 years. Elucidation of the complete nucleotide sequence of the human genome did not lead to immediate understanding of gene regulation and the genetic basis of human disease. Similarly, mapping the activity of every neuron and the complete ‘connectome’ (connections among all neurons) is a significant, but far from sufficient step, towards understanding the principle of information processing and the neural-circuit mechanisms underlying normal and aberrant brain functions. It is therefore critical that the funding of the mega brain projects does not affect support to the numerous small laboratories around the globe that are making steady progress in elucidating neuronal and neural-circuit mechanisms underlying various brain functions. Nevertheless, there is little doubt that the BRAIN Initiative will help accelerate the development of new technologies that allow large-scale measurements of neuronal activities at high spatiotemporal resolution, mapping of neuronal connectivity with increasing efficiency, and more effective brain–machine interfaces to probe the dynamics of brain activity and manipulate the output of brain circuits.
PREMATURE SUPERCOMPUTER SIMULATION?
In the Blue Brain Project, the forerunner of the current HBP, European scientists led by Markram used existing knowledge of neuronal types and their synaptic connections to construct a computer model that simulates a mouse cortical column (< 1 million neurons), the functional module of the neocortex. A much more grandiose goal is proposed in the HBP [2]: a global information and computing technology platform for neuroinformatics, brain simulation, and supercomputing that will allow neuroscience data from all levels (from genes, molecules and neurons, up to neural circuits, human cognition and behavior) to be integrated into unifying models of the brain. It is proposed that clinical data could also be integrated into computer models of diseases that may help physicians develop techniques for diagnosing brain diseases, understand their disease mechanisms and search for new treatments. The resources associated with the HBP may indeed facilitate the development of supercomputing technologies, and some brain models may also help the development of new computing systems and robots based on brain architecture and circuitry. It is much less clear how supercomputer models of the brain, as proposed for the HBP [2], may help our understanding of brain functions and diseases. This is because our current knowledge about the neural circuitry underlying most brain functions and the way neural information is processed, stored and retrieved, remains highly rudimentary. The amount of guesswork required to set the parameters for supercomputer simulations of the brain will render the outcomes and conclusions unrealistic and unreliable, if not misleading. Interestingly, the BRAIN Initiative (and its earlier version of the ‘Brain Activity Map’), in the United States is precisely aimed at technologies that will eventually provide the information required for fruitful supercomputer brain simulations.
GLOBAL DISEASE BURDEN AND NEW STRATEGIES
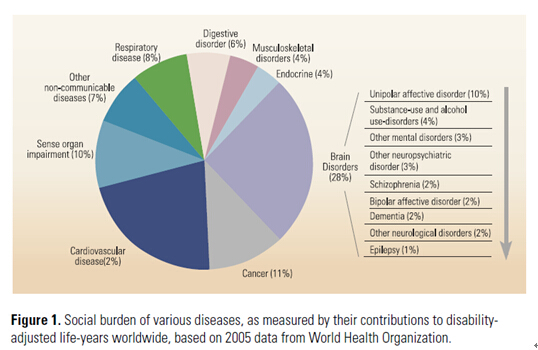
In comparison to the rapid advances in recent years of our understanding of the brain structure and function, the progress in finding effective treatments for all forms of brain disorders has been slow and the development of new drugs for neurological and psychiatric disorders by pharmaceutical companies has been disappointing. The high cost, low success rate and the long duration of clinical trials have resulted in major companies hesitating to invest in neurodrug development. Brain functions often involve neural circuits composed of diverse neuronal types in multiple brain regions, making it difficult to pinpoint specific loci for drug treatment. There is also a lack of suitable animal models for testing the action of drugs for psychiatric diseases. While efforts to identify potential drug targets will continue, and the hope for magic drugs for brain disorders remains, these drugs may not arrive in time to prevent the collapse of the medical care system in dealing with brain disease patients. This calls for new global strategies for dealing with the social burden of brain disorders, for which efforts have been initiated by multi-national programs [3] (see Figure 1).
Social burden of various diseases, as measured by their contributions to disability-adjusted life-years worldwide, based on 2005 data from World Health Organization.
The predominant theme of translational research (from bench to bedside) has been the development of pharmaceuticals that target disease loci. A rather unique feature of the brain is its structural and functional plasticity that allows the readjustment of its neural circuits upon physiological and physical stimulation or insults. Brain plasticity is at its highest in the early years after birth, when sensory and motor experience refine and consolidate neural circuits as we learn to feel, walk and speak, but substantial plasticity remains in neural circuits that enable life-long learning, memory and cognition, all of which involve dynamic and sometimes persistent modifications of the circuit structure and function. Substantial evidence now supports the notion that plasticity-based therapies using rationally designed physiological and physical stimulation offer new approaches in the treatment of brain diseases that are safe, cheap and applicable to large populations [5]. The drive for new neurotechnologies may facilitate the design of new tools for non-invasive brain stimulation with high spatiotemporal precision, and new brain–machine interface devices that effectively use the functional output of the brain to instruct the pattern of brain stimulation for correcting circuit dysfunctions.
Among the various brain disorders, neurodegenerative diseases, such as Alzheimer disease (AD) and Parkinson's disease (PD) have become an increasing menace to the aging society. Degeneration and death are the natural neuronal fate of an aging brain, resulting from life-long environmental insults and cumulated harmful gene mutations and damages. Treatment of neurodegenerative diseases should aim to protect neurons from extrinsic and intrinsic factors that cause their degeneration and such treatments are better applied early than late. Early diagnosis, followed by early intervention, is likely to be the most effective approach in dealing with neurodegenerative diseases. Identification of early biomarkers of the diseases, either biochemicals or cognitive functional deficits, followed by the rational design of an early intervention program, may help to slow down the process of neurodegeneration. A delay in the onset of severe AD and PD symptoms by 10 years, for example, would greatly relieve the disease burden on society. It is of interest to note, however, that the prospect of effective brain disease therapies through early intervention lies as much in developing innovative programs in our medical care systems and public health institutions as in the technological advance made in neuroscience laboratories [3, 6].
For the neuroscience community, the year 2013 will be marked by a wave of global awareness of the importance and urgency of brain research, as reflected by the mega projects initiated in the USA and Europe. Looking back, there has been no shortage of government interest in promoting neuroscience research. One would hope that these new initiatives do not stay in the laboratory, but spread to programs that directly and effectively provide medical care for patients with brain disorders.
本网站所有内容来源注明为“williamhill asia 医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于williamhill asia 医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“williamhill asia 医学”。其它来源的文章系转载文章,或“williamhill asia 号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与williamhill asia 联系,williamhill asia 将立即进行删除处理。
在此留言










#ATL#
54
#Nat#
46
#脑科学#
45
传说中的中国脑计划
135
NSR,高大上期刊,第一期上的。不过,还是没搞清楚蒲先生想说什么?
187